Kota KodamaA comparative study of Japanese, US, and French regulation and social system of AI used in medicine医学で使用されるAIの日米仏の規制と社会システムに関する比較研究 Étude comparative de la réglementation et du système social japonais-américain-français de l'IA utilisée en médecine 2021/01/13 AbstractThis research aims to find answers to the following questions: What are the main differences between the governmental policies used in terms of utilizing AI in medical devices and therapeutic applications? How will the entire social system change when a new technology called mHealth is introduced into society? この研究は、以下の質問に対する答えを見つけることを目的としています。 医療機器および治療用途でAIを利用することに関して各政府の政策や規制の主な違いは何ですか? mHealthという新しい技術が社会に導入された際に社会システム全体がどのように変わるのか? BackgroundThe level of interest about the artificial intelligence applications is continuously rising worldwide especially in the health care applications(1). Medical devices are an essential part of the healthcare sector, and there is a growing interest in utilizing machine learning (ML) and deep learning (DL), generally called Artificial Intelligence (AI) technologies to enhance the accuracy and adaptability of the medical devices based on consumers' data. The global medical devices market is also continuously growing. According to the Medical Device Market Report published by Lucintel in April 2018(2), the medical devices market is expected to have a 4.5% compound annual growth rate CAGR from the year 2018 to the year 2023. It is estimated that the market will grow to reach $409.5 billion by the year 2023, creating investment opportunities and prospects for advancements in this field. Current status & challenges of adopting AI & ML to medical devices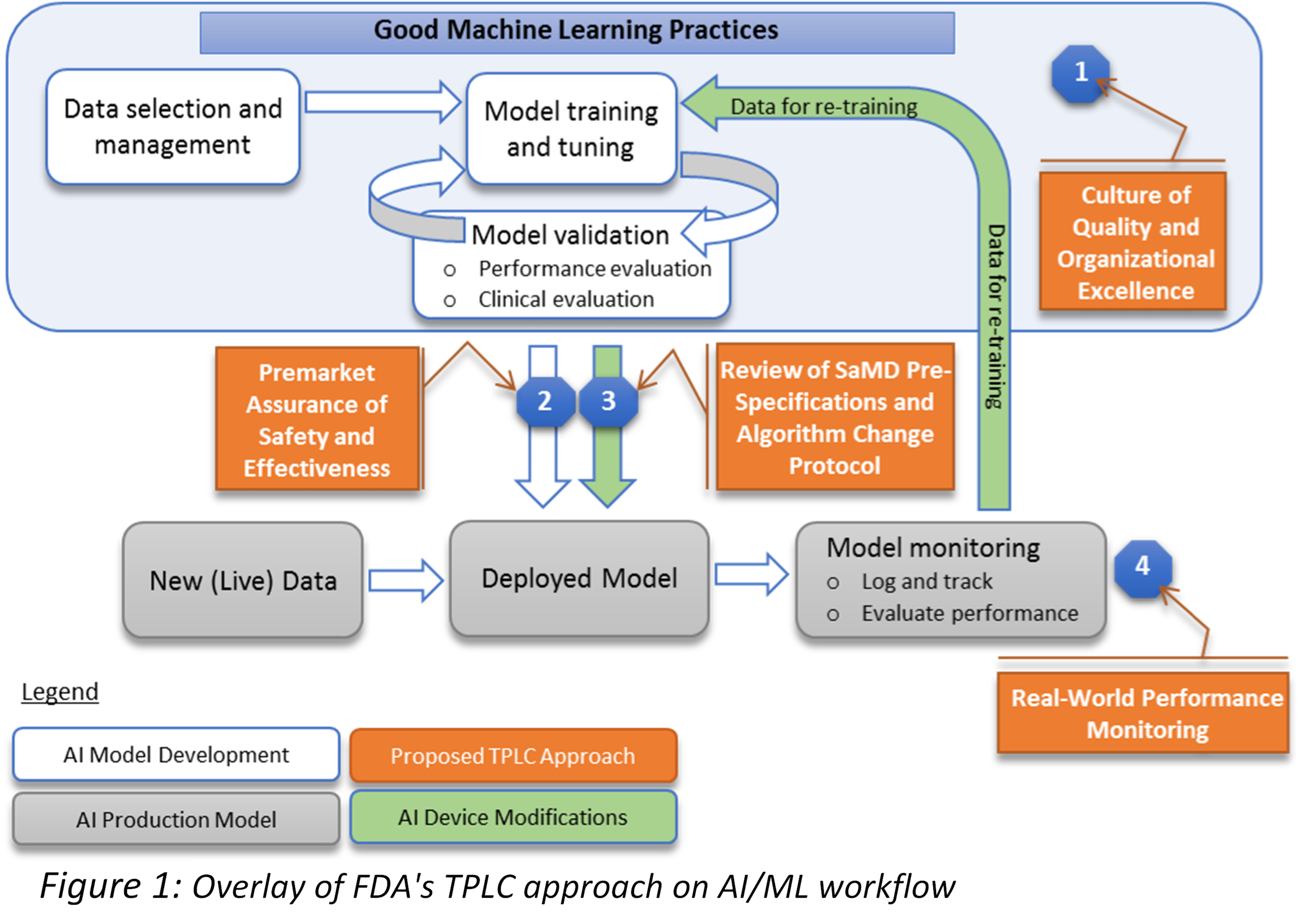
The role of artificial intelligence is growing rapidly especially in the medical devices field. With the ability to save time, efforts and huge cost cut in the development process, AI shall create a massive spread among the medical devices manufacturers especially with the proven abilities of artificial intelligence and machine learning to bring unprecedented functions such as the early detection for diseases, automatic diagnostics, continues development, and many other functions. However, the regulation of utilizing the artificial intelligence in the medical devices tends to be complicated in most of the countries, due to the nature of the AI and the fact that the algorithms used are continually developing and adapting based on the existence of new data sets. This challenge makes it difficult for the legislation authorities to regulate such a complex operation that evolves and changes over time, also creates difficulty for the healthcare companies to adapt to the regulations. We believe that this is a core factor that hinders innovation and adoption in this field. In order to overcome this challenge, several governments have already started to work on special legislation system for the drug discovery using AI. One of the main initiatives in this field is the FDA initiative that has started in April 2019 to craft new legislation and framework to regulate the modifications to artificial intelligence and machine learning software utilized in the healthcare industry. As described in Figure 1, a new approach of the total product lifecycle (TPLC) regulatory has been created which is necessary for the AI/ML-based medical devices that have the ability to adapt and improve by using users’ data. This approach shall enable reviewing and monitoring the software products from the pre-market stage up to the post-market stage, with continues revision for the creators’ internal policies and operational excellence levels(3). ObjectivesThe first objective (Regulatory Science) is to compare the regulation of utilizing the AI technologies in the healthcare especially in the medical devices field between different countries. Also, to explore the ability to solve the dilemma of continues changing algorithms by utilizing Algorithm Change Protocols (ACP) as described in the recent publications of the FDA. ACP is a clear description and protocol of methods and restraints that the AI companies must utilize in order to control the risk that could result from different types of modification to the algorithms or software. The protocol explains a detailed delineation of the procedures to be followed when handling the new data so that way the modifications achieve its goal and the algorithm could remain safe in a partially controlled environment. The research shall create a framework to identify the factors accepted as a protocol in each country, focusing on mobile health (mHealth), which is being introduced in this area. Identifying this framework shall boost the legislative efforts in creating accepted protocols for the AI medical devices, also will boost the innovation and investment in the medical companies that adopt the AI approach. One of the growing applications for AI is a therapeutic mobile application(4) that is expected to adapt to a variety of mental disorders, including dementia, bipolar affective disorder, schizophrenia, and mainly depression. According to the World Health Organization, depression alone affects at least 300 million persons globally, and it costs the USA hundreds of billions of dollars annually as an economic loss. Even with the existence of treatment options and social services, the majority of the people affected by mental disorders tends to avoid having a hospital record to maintain their private identity and avoid being associated with such illnesses. Therefore, several technology companies have been developing artificial intelligence based mobile phone applications to provide support for the mental health patients while maintaining patient privacy. These solutions provide the patient with a very high value which is being available around the clock to listen to them or to provide any support or advice needed. Due to the fact that those solutions are mainly based on artificial intelligence and machine learning, it has created the same concerns by the legislative authorities’ due to its continually changing nature and the sensitivity of the core value that it provides. The study aims to discover the main differences in the regulations that govern the authorization of such therapeutic applications between France, Japan, and the USA. The study also will define the main challenges that face the developers of the therapeutic applications in terms of getting the necessary approvals and being compatible with the rules and regulations the countries previously mentioned. The reason why we are focusing on France instead of the entire EU is due to the design of the mHealth approval system and insurance reimbursement system. Although Japan and the U.S. have a single mHealth approval system and insurance reimbursement system, the EU operates only one mHealth approval system for the entire EU region, while the insurance reimbursement system is left to each country in the EU. Therefore, in order to compare both the approval and reimbursement systems for mHealth, this study chose France instead of the EU as the research target in order to simplify the comparison. Since the institutional design related to mHealth and AI is still immature in all countries, we initially planned to conduct interviews with regulatory officials in each country to make comparisons, including their future policies. However, due to the recent COVID-19 situation, we decided to change our research plan and conduct a comparative study using open data published by regulatory authorities. The second purpose (social system) is to set the analysis target to the whole society and to consider the social system in which mHealth is highly useful, to help the sound development and use of mHealth. If mHealth's usefulness cannot be properly considered, it will be a situation where it will not be put into practical use, although it is a potentially useful technical concept. There are situations in which it may be considered that medical evaluation alone is not useful unless it exceeds existing medical care, and research from a social perspective is necessary. In order to examine the transformability of the healthcare system by mHealth, we model the attention part of the healthcare system and examine the effect of mHealth by the system dynamics (SD) method. The model is developed by applying the existing SD model (Lyon 2016, Chen 2018) of previous research. Figure 2 shows the status of examination of issues in screening for depression (major depressive disorder). The implementation of mHealth changes how screening efficiency, positive predictive value (PPV), negative predictive value (NPV), treatment efficiency, and treatment adequacy at the time of screening shown in the figure change examine how suicide rates, patients' quality of life (QOL), lost productivity due to depression (major depressive disorder), and their impact on health care costs and capacity.
The third objective (Science Communication) is to know how the general patient thinks of the AI used in medicine. Although the word “AI” can be captured in a very vague sense, AI currently applied in medicine is only clinically applied using data science technologies such as machine learning and deep learning. Therefore, it does not mean that a computer that is associated with a word called artificial intelligence can spontaneously make a decision such as a diagnosis. Perhaps, even general clinicians are considered to have such misunderstandings, so it is thought that the gap between recognition with general patients is even greater. However, when conducting related technology development, it will be very important to carry out development while grasping such general citizens' needs, because they are potential customers doing development, they have true unmet medical needs. We will conduct a web questionnaire survey on the medical application of AI technology to general citizens in mainly in Japan and France (included EU and the US). Through this research, I would like to consider the better future of medical and AI technology. Research questionThe research aims to find answers to the following questions with utilizing the scientific approaches:
Finding answers scientifically for the above-mentioned questions shall provide a proven framework that describes the main factors that increases or decreases the adoption rate of the AI technology, which will eventually could be utilized in boosting the adoption rate of this field, which would be an added value for the whole healthcare sector as well as it would have a positive economic impact. Research approach and methodologyThe study mainly shall follow a comparative research approach between France, Japan, and the USA covering both folds of the policy comparison approaches(5):
The study shall utilize quantitative approaches in the data gathering and analysis process. Multiple simulations with system dynamics utilizing the collected data will be conducted. Through these simulations, we will consider what form of social implementation will facilitate the degree of utilization of AI technology in mHealths in the first place. The results will be used to identify the prospects for further use of the technology in the future and the main challenges faced by companies using AI in pharmaceutical regulations. In addition, we will gain a better understanding of what key legislative changes mHealth companies are recommending to boost innovation in the AI medical field. Also, we will conduct an online survey measuring the perspective and acceptance rate of the public for utilizing AI in the healthcare field. Notes(1) McCarthy, J. (2007). What Is Artificial Intelligence? Stanford University, Stanford, CA. Retrieved from http://jmc.stanford.edu/articles/whatisai/whatisai.pdf(2) Medical Device Market Report: Trends, Forecast and Competitive Analysis, April 2018, Lucintel: insights that Matter, https://www.lucintel.com/toc/medical-device-market-2018.aspx (3) Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML), US Food & Drug Administration, April 2019 (4) Personalized “deep learning” equips robots for autism therapy, Machine learning network offers personalized estimates of children’s behavior, Becky Ham | MIT Media Lab, June 27, 2018, http://news.mit.edu/2018/personalized-deep-learning-equips-robots-autism-therapy-0627 (5) Charles Ragin (1987): The Comparative Method: Moving Beyond Qualitative and Quantitative Strategies. Berkeley: University of California Press. |
 |
Recherche |  |
FFJ Research Statement |  |
Kota Kodama |
| Inscrivez-vous à notre Lettre en cliquant ici |
*En cas de problème, vous pouvez aussi vous inscrire en envoyant un mail à sympa@ehess.fr, avec pour titre "subscribe ffj_french_news".






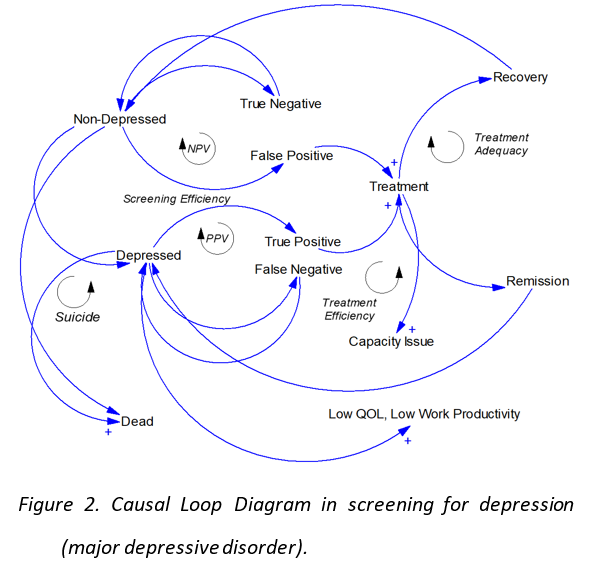 Considering the current situation of different administrative guidance in Japan, the United States and Europe, the current QOL, cost-effectiveness, regulation, insurance system, etc. are compared in three regions of Japan, the United States and Europe in order to examine the impact of social systems on mHealth development. And clarify differences in social infrastructure. The effect of mHealth will be examined for each region by the system dynamics incorporating these differences.
Considering the current situation of different administrative guidance in Japan, the United States and Europe, the current QOL, cost-effectiveness, regulation, insurance system, etc. are compared in three regions of Japan, the United States and Europe in order to examine the impact of social systems on mHealth development. And clarify differences in social infrastructure. The effect of mHealth will be examined for each region by the system dynamics incorporating these differences.